When optimizing catalysis in the lab, product selectivity and conversion efficiency are primary goals for materials scientists. Efficiency and selectivity are often mutually antagonistic, where high selectivity is accompanied by low efficiency and vice versa. Increasing the temperature can also change the reaction pathway. In a new report, Chao Zhan and a team of scientists in chemistry and chemical engineering at the Xiamen University in China and the University of California, Santa Barbara, U.S., constructed hierarchical plasmonic nanoreactors to show nonconfined thermal fields and electrons. The combined attributes uniquely coexisted in plasmonic nanostructures. The team regulated parallel reaction pathways for propylene partial oxidation and selectively produced acrolein during the experiments to form products that are different from thermal catalysis. The work described a strategy to optimize chemical processes and achieve high yields with high selectivity at lower temperature under visible light illumination. The work is now published on Science Advances.
Catalysts
Ideal catalytic processes can produce desired target products without undesirable side effects under cost-effective conditions, although such conditions are rarely achieved in practice. For instance, high efficiency and high selectivity are antagonistic goals, where a relatively high temperature is often necessary to overcome the large barrier of oxygen activation to achieve high reactant conversion. Increasing the functional temperature can also lead to overoxidized and therefore additional byproducts. As a result, researchers must compromise between selectivity and efficiency. For instance, a given molecule typically requires diverse catalysts to generate different products, where each catalyst has different efficiency and selectivity. To circumvent any limitations, they can use surface plasmons (SPs) to redistribute photons, electrons and heat energy in space and time. In this work, the team used propylene partial oxidation as a model system and a plasmonic hierarchical nanostructure as a catalyst. Using the setup, they showed how the excitation of SPs simultaneously improved the selectivity and conversion efficiency to simultaneously activate high yields of product with high selectivity at low temperatures. The catalysts contained well-defined copper oxide nanocrystals (Cu2O) with good catalytic activity; further activated using plasmonic gold nanoparticles (Au-Cu2O). Zhan et al. used visible light illumination to show an 18-fold increase of propylene conversion, while the selectivity of acrolein increased approximately by 50 to 80 percent during the experiments.
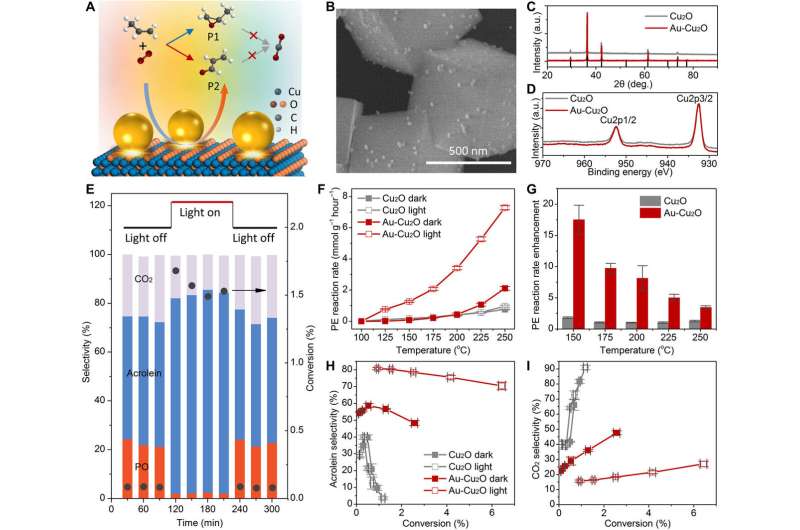
The experimental system and characterization of catalysts relative to illumination.
The scientists varied the wavelength of the setup and used silicon dioxide shells to isolate the electronic effects to then develop a computational model to understand the experimental process. Zhan et al. determined how plasmonic effects such as energetic electrons and thermal feeds confined at the nanoscale provided different effects on reaction selectivity to regulate the reaction pathway and selectively produce acrolein or eliminate consecutive reactions. The team conducted partial oxidation of propylene in a quartz microreactor at atmospheric pressure for simultaneous temperature control and illumination. They chose this reaction due to its commercial value. Zhan et al. used a 300 W Xenon lamp filtered to exclude the ultraviolet region as a light source with a total intensity of 200 mW/cm2. They identified acrolein, polypropylene oxide and carbon dioxide as the dominant reaction products. Using X-ray diffraction and X-ray photoelectron spectroscopy, they confirmed the crystal structure and surface composition of cubic copper oxide (C-Cu2O). They then conducted the catalytic experiments under a variety of temperatures with or without illumination. In the absence of illumination, the measured reaction rate of propylene on C-Cu2O was consistent with previous reports. Upon illuminating gold-based Au-Cu2O, the propylene conversion increased greatly. To determine the plasmonic enhancement, Zhan et al. divided the property of the catalyst under illumination by that without illumination to determine plasmonic enhancement.
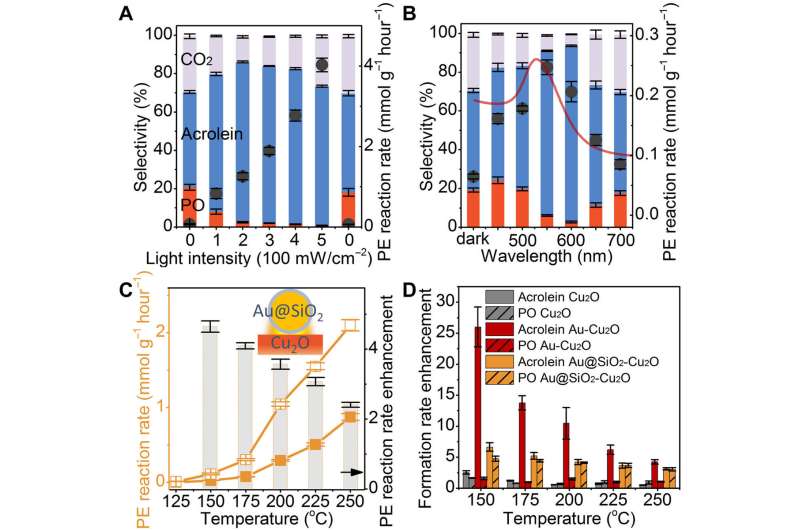
Light intensity and wavelength dependent experiments
The scientists then noted the catalytic performance as a function of light intensity with a supralinear dependence that formed a hallmark of the chemical reaction driven by surface-plasmon induced energetic electrons. However, in complex systems, it is difficult to use this as sufficient evidence to determine the energetic electron process. The unique propylene oxide selectivity depended on the wavelength of the incident light and in this instance resulted from various contributions of local heating vs. energetic electrons. To discern energetic electrons from local heating in plasmonic crystals, Zhan et al. coated the gold nanoparticles (NPs) with 5-nm thick silica shells to reduce electron transfer while allowing local heating. Using transmission electron microscopy, cyclic voltammetry and Raman spectra, the team proved the absence of pinholes in the shell. The charge transfer process was further inhibited by the 5-nm silicon dioxide shell. The scientists then used the gold silicon dioxide copper oxide (Au@SiO2-Cu2O) hierarchical structure as a catalyst and conducted the experiments at various temperatures with or in the absence of illumination.
Discerning local heating effects
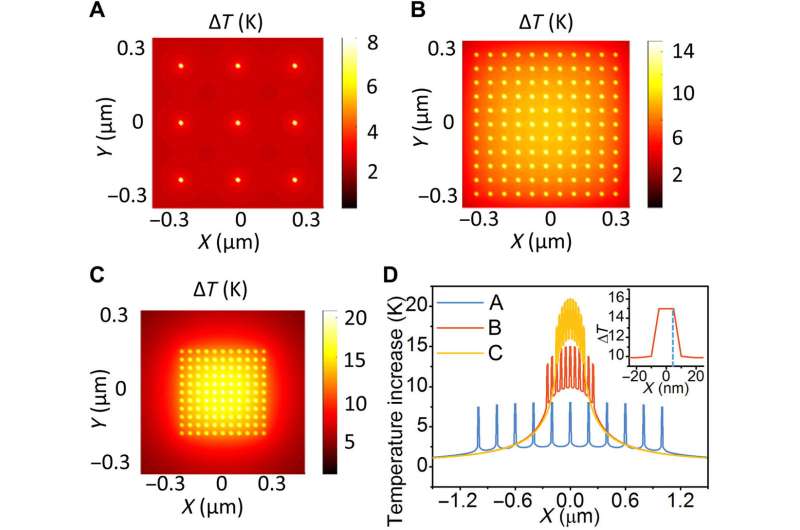
The team also conducted experiments to confirm the existence of nanoconfined thermal fields. To accomplish this, they calculated the temperature distribution using a conventional macroscopic model. Zhan et al. then considered the interfacial thermal resistance between the particle and the surrounding medium, while also considering the collective heating effect relative to the particle density. They then considered the thermal effect of gold nanoparticles assembled on a copper-oxide surface with various particle densities. At low particle density, the team observed high temperatures to be localized in the vicinity of the particles with limited temperature increase in the surrounding medium. At high particle densities, the temperature was no longer localized, and instead the surrounding medium showed a higher temperature.
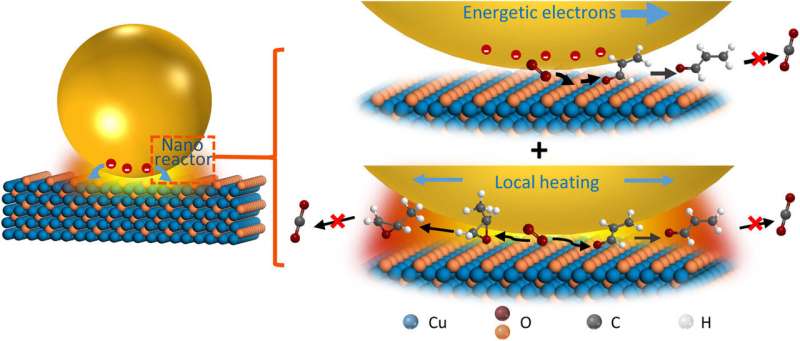
Outlook
In this way, Chao Zhan and colleagues showed a unique environment created by surface plasmons to greatly enhance the conversion and regulate the selectivity of propylene-selective oxidation. They credited the outcome to energetic electrons coupling with nanoconfined thermal fields. The phenomenon acted on the chemical reaction through diverse ways to result in different outcomes. The plasmonic reactor coupled the energetic electrons and nanoconfined thermal fields to promote the conversion rate and regulate selectivity concurrently compared to competitive regulation. The plasmonic reactors also had diverse effects on chemical reactions and regulated the reaction pathways by reducing consecutive reactions. Plasmonic nanostructures can be made mutually selective and efficient, suggesting a paradigm applicable across a range of catalytic processes. The surface plasmons offer a new mechanism to conduct catalytic reactions and enable a more efficient use of solar energy or visible light to drive chemical reactions.
Zhan C. et al. Plasmonic nanoreactors regulating selective oxidation by energetic electrons and nanoconfined thermal fields, Science Advances, DOI: 10.1126/sciadv.abf0962
Tyo E. C. and Vajda S. et al. Catalysis by clusters with precise numbers of atoms. Nature Nanotechnology, doi.org/10.1038/nnano.2015.140
Baumberg J. J. et al. Extreme nanophotonics from ultrathin metallic gaps. Nature Materials, doi.org/10.1038/s41563-019-0290-y
Science Advances
,
Nature Nanotechnology
,
Nature Materials