The extracellular matrix (ECM) including three-dimensional (3D) network and bioelectricity can profoundly influence cell development, migration, and functional expression. In a new report now published on Science Advances, Tong Li and a research team in chemistry, nanotechnology, bioelectronics and advanced materials in China, developed an electromechanical coupling bio-nanogenerator abbreviated bio-NG inspired by biophysical cues of the extracellular matrix. The device contained highly discrete piezoelectric fibers to generate piezo potential of up to millivolts to provide in situ electrical stimulation for living cells.
The unique 3D space within the bio-NGs provided an ECM-like environment to promote cell growth. The bio-NGs effectively promoted cell viability and development to maintain its specific functional expression. Researchers expect the new and advanced bio-NGs to mimic the complexity of the extracellular matrix and provide a physiologically relevant in vivo biological system. The device effectively promoted cell viability and development to maintain its specific functional expression. Li et al. expect the new and advanced version of bio-nanogenerators to provide a physiologically relevant in vivo biological system to replace inaccurate 2D systems and animal models.
Guidance for cells
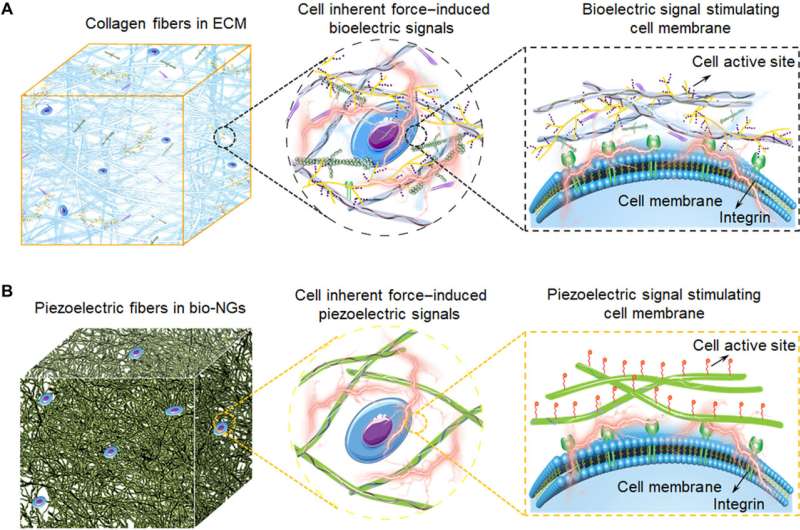
In this work, Li et al. outlined a practical strategy for wireless electrical stimulation of cells and tissues to repair and sustain cell function. Bioelectricity is a biophysical cue that provides guidance for cell growth and differentiation during embryonic development and tissue regeneration. Endogenous bioelectricity exists in the cytoplasm and extracellular space, providing scientists a resource for electrical stimulation of excitable cells and regulating cellular activity for biomedical applications. Most treatment methods require an external energy input and wire connection to apply external electrical pulses through implanted microdevices. Recent developments in nanotechnology have allowed electrode-less and battery-free treatments, which include the use of nanogenerators for brain stimulation, hair regeneration and wound healing. However, most of them require a well-accepted solution to electrically stimulate the functional cells. Li et al. were therefore inspired by the biological function and microstructure of collagen fibers in the extracellular matrix to form bio-NGs composed of highly discrete piezoelectric electrospun fibers to provide cells with a physically relevant microenvironment. The bio-NG-cell interaction applies to in vivo environments to reduce inflammation, induce hepatocyte proliferation, and accelerate angiogenesis, as well as promote liver repair.
Forming the bioinspired electromechanical bio-NGs.
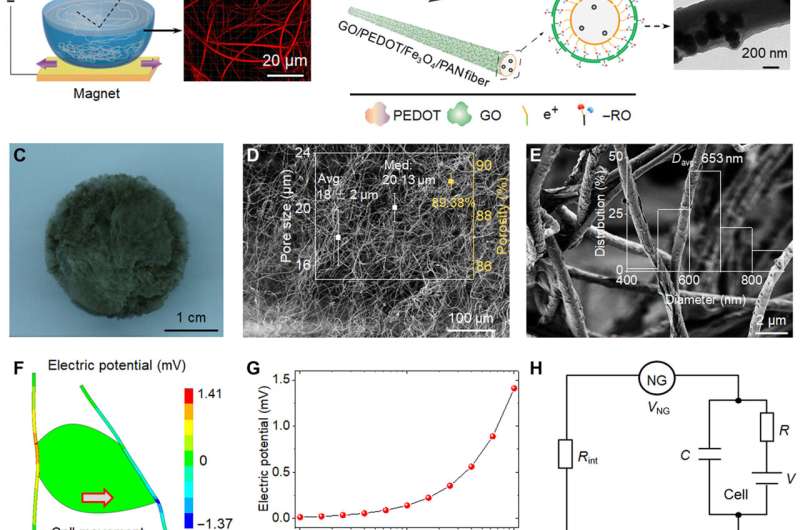
During the experiments, the research team introduced iron oxide magnetic nanoparticles into polyacrylonitrile to prepare highly discrete fibers for use as a magnetic-assisted electrospinning device. During electrospinning, the setup enabled the formation of scaffolds with well-interconnected pores and discrete fibers for cell-free migration. To prepare a closer-to-in-vivo microenvironment, the team also imparted bioelectricity as a biophysical cue. To accomplish this, the scientists developed a target scaffold to promote cell interaction and adhesion with fibers. The electromechanical coupling of bio-NGs assembled by the scaffold promoted the transmission and communication of signals between cells to mimic the bioelectric effects of collagen fibrils or fibers in the extracellular matrix. The team simulated and studied the piezoelectric potential generated from cell force in bio-NGs using finite element analysis. To accomplish this, they applied a load force to the cell-fiber contact and first measured the piezoelectricity of a single fiber within bio-NGs using piezoelectric force microscopy. The experimental voltage signals validated the theoretical piezoelectricity of the bio-NGs.
Characterizing the bio-NGs and regulating cell activity
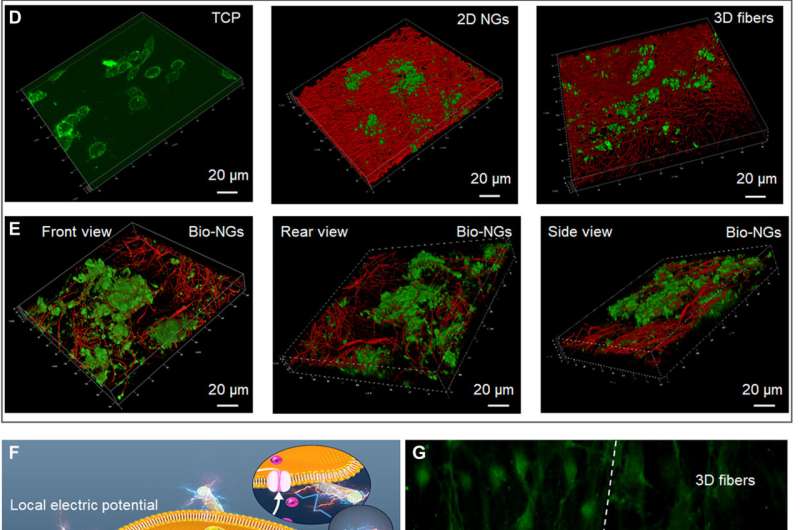
To investigate the information of the fibers in bio-NGs, the team used Fourier Transform Infrared (FTIR) and X-ray diffraction (XRD) spectra. They then studied the thermodynamic properties of the piezoelectric fibers in bio-NGs using differential scanning calorimetry (DSC) thermograms and conducted cyclic voltammogram studies to test the charge storage and transmission properties of the piezoelectric fibers in bio-NGs. The team then tested the compressive resilience and mechanical properties of the fibers by first forming cylindrical shapes of them and compressing the scaffolds to understand the excellent resilience of the constructs. The mechanical properties and resilience of the fibers ensured the bio-NGs could effectively maintain a large enough pore size and stable 3D growth microenvironment for cell movement and growth. The team also investigated the NG-cell interaction in 3D space with two different cell lines including
retinal ganglion cell 5 (RGC5) and primary hepatocytes. The cells contained voltage-gated calcium channels in their membranes and others were motile cells with high metabolic functions. Using two-dimensional nanogenerators (NGs) and non-piezoelectric 3D fibers the team studied the effects of 3D space and electrical stimulation on cells. The data showed how the bio-NGs could provide a biofriendly cell culture microenvironment for further experiments.
Promoting in vivo liver repair with bio-NGs
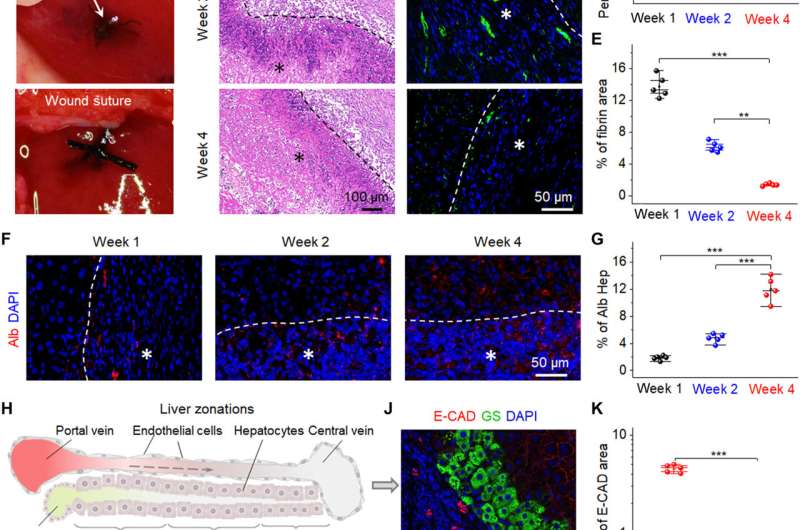
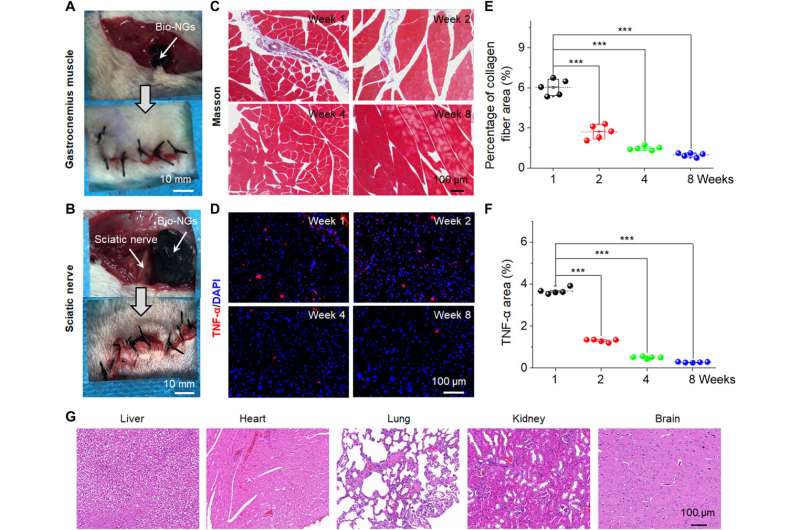
The scientists then implanted the bio-NGs into an area of liver injury relative to hepatocyte regeneration to reflect their practicality. To accomplish this, they used Sprague-Dawley rats to induce liver injury. After four weeks of implanting the bio-NGs, the team removed the implants and studied inflammation using histology staining. They noted mild inflammation in the first week, which improved by the second week and reduced to normal levels by the fourth week. All other organs did not show deformation or abnormal lymphatic cell invasion to indicate good health conditions without systematic side effects. The observed regenerative process highlighted a new blood circulation system that formed inside regenerated liver tissue to suggest the interaction of bio-NGs with cells to reduce inflammation and promote tissue repair.
Long term stability and biocompatibility of bio-NGs in vivo
The NG-cell interaction efficiently promoted cell viability and maintained its functional expression in vitro and in vivo to provide a treatment strategy for clinical trials. For tissue regeneration, it is most effective to directly transplant functional cells into the damaged site in vivo. For additional studies, the team implanted the bio-NGs into the gastrocnemius muscle area around the sciatic nerve of rats to detect the stability of the bio-NGs in vivo. Li et al. then removed the implants after eight weeks and analyzed inflammation to show good biocompatibility of bio-NGs for prolonged periods of time in biological environments without any systemic side effects. The constructs are promising as implants for in vivo regenerative repair.
Outlook
In this way, Tong Li and colleagues developed extracellular matrix-like electromechanical coupling bio-nanogenerators (bio-NGs) to regulate cell activity and maintain its specific functional expression. The product created a local voltage potential to stimulate living cells as long as they remained motile. The unique environment facilitated cell culture in bio-NGs to trigger the opening of ion channels present in the cellular plasma membrane to achieve electrical stimulation at the single-cell level. The process offers great potential for bioelectronic medicine and cell-targeted local electrical impulses. The new method can replace inaccurate 2D systems and time-consuming animal models to provide a biomimetic, physiological microenvironment for accelerated tissue regeneration and bioinspired electronic medicine.
Tong Li et al, Cell activity modulation and its specific function maintenance by bioinspired electromechanical nanogenerator, Science Advances (2021). DOI: 10.1126/sciadv.abh2350
Qiang Zheng et al, Self-powered cardiovascular electronic devices and systems, Nature Reviews Cardiology (2020). DOI: 10.1038/s41569-020-0426-4
Science Advances