Nanoparticles are actively employed in medicine as contrast agents as well as for diagnosis and therapy of various diseases. However, the development of novel multifunctional nanoagents is impeded by the difficulty of monitoring their blood circulation. Researches from the Moscow Institute of Physics and Technology, the Shemyakin-Ovchinnikov Institute of Bioorganic Chemistry of RAS, Moscow Engineering Physics Institute, Prokhorov General Physics Institute of RAS, and Sirius University have developed a new noninvasive method of nanoparticle measurement in the bloodstream that boasts a high time resolution. This technique has revealed the basic parameters that affect particle lifetime in the bloodstream, which may potentially lead to discovery of new, more effective nanoagents to be used in biomedicine. The results of the study have been published in the Journal of Controlled Release.
Clinical applications of nanoparticles (NP) require an accurate analysis of their behavior in vivo, in particular the duration that the NP stay in the bloodstream. It is the parameter that determines if there is enough time for the NP to spread throughout the body, reach their therapeutic target (e.g., a tumor), and bind to it. Alternatively, the excessively long circulation time may lead to the accumulation of the particles in healthy tissues, thus increasing their side toxicity.
NP circulation in the bloodstream is usually studied through drawing blood samples and measuring the nanoagents content. “The problem of such techniques is that particles are often cleared from the bloodstream within mere minutes so the researcher is only able to take two or three blood samples, which is insufficient for the analysis,” commented study co-author Maxim Nikitin, who heads the Nanobiotechnology Lab at MIPT.
Apart from that, repeated blood drawing is stressful for the organism and may indirectly affect particle circulation. The new noninvasive methods of monitoring particle activity in vivo are therefore crucial for the development of nanomedicine.
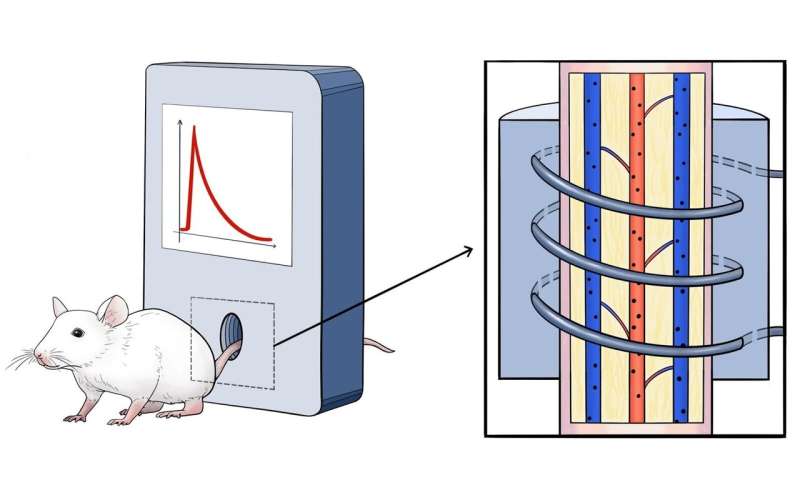
The researchers used the magnetic particle quantification (MPQ) method developed by them to take noninvasive measurements of the blood particle kinetics. Mice or rabbits’ tails were placed into the detection coil of the MPQ reader, then the animals were injected with the nanoparticles, and the NP concentration in their tail veins and arteries was monitored in real time. This technology may be used with humans as well, e. g., via hands or fingertips placed into the detection coil.
The new method offers a noninvasive way of obtaining unique information on particle kinetics that is also simpler than the traditional approaches. It enables further exploration of what might influence particle behavior in the animals’ bloodstream.
The researchers investigated three groups of factors including the particle physic-chemical properties, the particularities of their administration, and the state of the animal’s body. The smaller-sized negatively charged NP injected in higher doses remained in the bloodstream longer. It has also been found that if particles are injected into blood repeatedly, the circulation of subsequent particle doses becomes significantly prolonged.
“There are similar cases in clinical practice when a patient is initially injected with nanoparticle MRI contrast agents (magnetic particles) and then with the therapeutic NP such as liposomes carrying drugs. We have shown that particles can affect each other, which may influence the treatment,” said the study’s author, Ivan Zelepukin, a researcher at the Russian Academy of Sciences Institute of Bioorganic Chemistry and MIPT.
What appeared to be one of the key aspects was the NP-injected organism’s state. For instance, the particle circulation could significantly vary among mice of different genetic strains. Notably, this difference was evident only for small 50-nanometer particles but not for bigger nanoagents. Besides, if the animal had a large tumor, small NP were eliminated from the blood more rapidly; the larger the cancer tumor was, the less time the blood clearance took.
The researchers assume this may be linked to dynamic changes in the immune system and its increased ability to recognize foreign matter in response to pathology. These findings draw attention to the importance of considering the impact of the organism’s condition on the effectiveness of nanoparticles in designing the optimal nanodrugs—an aspect that has traditionally been ignored.
“This is the first time such a comprehensive study of NP with extremely high clearance rate has been carried out. It would have been impossible without the methodology that is being developed at the General Physics Institute of RAS. The MPQ technique combines high sensitivity, high time resolution, and quantitative accuracy. Apart from that, it is noninvasive and it enables detection of NP content almost in real time,” said Petr Nikitin, a co-author of the study and the head of the Biophotonics Lab at the General Physics Institute of RAS.
“Our method allowed us to detect new circulation patterns and obtain a great amount of valuable information. For example, we have found out that animals had different particle dynamics depending on their immune status, presence of tumors, etc. Meanwhile, the advanced methodology required much fewer animals for the study. This is essential not only in terms of time and finances but also the ethics of animals’ treatment in line with the principle of the 3Rs (Replacement, Reduction and Refinement). We assume that a deeper understanding of the underlying mechanisms may considerably facilitate the rational design of nanomaterials with advanced surface functionality and superior pharmacokinetics for the next generation diagnostics and therapeutics.”
Ivan V. Zelepukin et al. Fast processes of nanoparticle blood clearance: Comprehensive study, Journal of Controlled Release (2020). DOI: 10.1016/j.jconrel.2020.07.014
Journal of Controlled Release
Moscow Institute of Physics and Technology